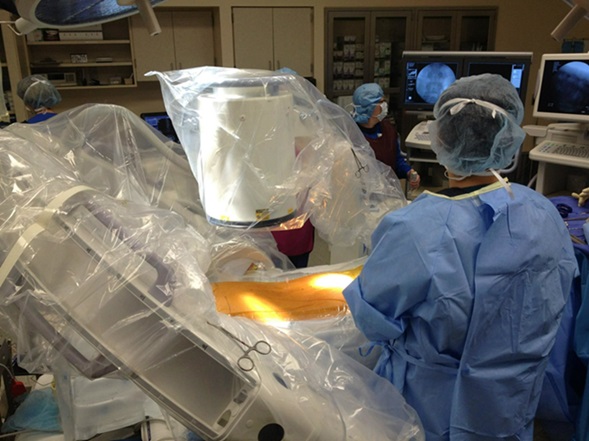
Inspired Spine CMO Dr. Hamid Abbasi performs one of more than 1,700 OLLIF procedures. The procedure has proven effective in hundreds of patients with a high BMI.
BURNSVILLE, Minn., Nov. 29, 2023 /PRNewswire/ — Inspired Spine has announced the formation of two new Centers of Excellence focused on providing complex spine care, including fusion surgery, to patients with high Body Mass Index (BMI) in rural areas of Minnesota and Kansas, a groundbreaking turn in modern spine health management.
In a remarkable display of dedication, Dr. Hamid Abbasi, a renowned neurosurgeon and the driving force behind Inspired Spine, recently performed an extraordinary feat by traveling to Hutchinson, Kansas to perform eleven spinal surgeries in just two days. Notably, five of these patients had a BMI over 40, with the highest BMIs being 54 and 64. All eleven operations were performed with unprecedented success and the patients were able to walk back and forth in the comfort of their own home within two days.
Traditionally, patients with a BMI above 40 face significant challenges in seeking elective surgeries due to societal norms and medical recommendations against such procedures citing high risks. Inspired Spine’s innovative Oblique Lateral Lumbar Interbody Fusion (OLLIF) technology and comprehensive surgical protocols have dramatically minimized associated risks for patients requiring spinal fusion surgery. Many of them have become non-ambulatory due to spinal problems and have no alternative. The benefits of OLLIF now impressively outweigh the risks.
This advancement comes at a crucial time for patients with high BMI, who often face a debilitating cycle of weight gain and deteriorating health due to the lack of mobility and associated comorbidities, such as cardiovascular decline. In response, Inspired Spine has established specialty medical centers accessible to patients from across the United States that provide advanced and life-changing care.
With a ten-year track record of successfully treating patients with a high BMI with the OLLIF procedure. This breakthrough has opened new doors to patient care that many thought were permanently closed. Despite the complex nature of these operations, patients are typically discharged only one or two nights after surgery and can return home within a few days – undeniable proof of the high efficiency and effectiveness of the treatment.
To underline this success, the publication of Inspired Spine’s OLLIF high BMI paper shows the transformative effects of this procedure. “We are proud to bring our innovation to rural Kansas,” said Dr. Abbasi. “The impact is already being felt with a series of successful surgeries performed in environments that would not normally be suitable for traditional spine surgery. Many of these patients require higher levels of care such as ICU or blood transfusions and intensive medical care due to the invasiveness of traditional surgeries.”
The human dimension of these successes is vividly reflected in patient testimonials. A recent patient from North Carolina who traveled to Inspired Spine of Kansas for the OLLIF procedure shares her experience: “I never thought relief was possible until I found Inspired Spine. The OLLIF procedure not only gave me back my mobility, but also gave me a new lease on life. The care I received in Kansas was exceptional, and I am grateful for the team at Inspired Spine.” Her story, along with her moving testimony and images, underscores the critical importance and positive results of the Centers of Excellence at Inspired Spine.
Inspired Spine is a total spine care provider dedicated to improving patients’ quality of life with revolutionary procedures and technologies. Our commitment to advancing spine health, grounded in innovation, has made us leaders in the field of minimally invasive spine surgery.
For more information about the OLLIF procedure and to read inspiring patient stories, visit www.inspiredspine.com or contact Amanda Armagost at aarmagost@islife.us
SOURCE Inspired Spine