Reduces time to weight bearing to two weeks from the current six to eight weeks
ATLANTA , Nov. 28, 2023 /PRNewswire/ — Shotel Medical today announced its Shotel™ Ankle Arthrodesis Nail System, which utilizes a novel device design for the treatment of end-stage ankle arthritis. It was used for the first time in Florida last month during a procedure in Delray Beach. Two weeks postoperatively, the patient was weight-bearing with the use of a CAM walking shoe, a significant improvement over the six- to eight-week time to weight-bearing offered by traditional ankle arthrodesis systems.
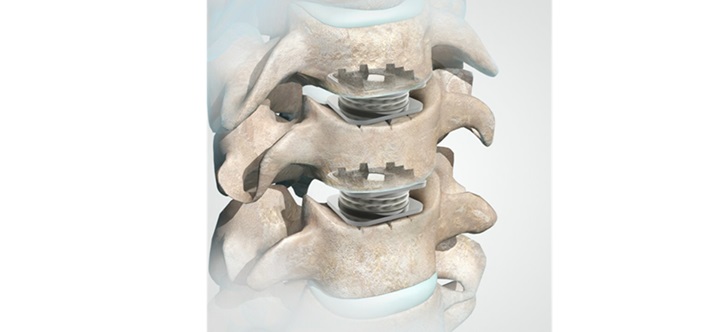
The patented Shotel Nail System, manufactured and distributed by BioPro Implants, differs from traditional ankle arthrodesis devices because its unique curved design allows for a minimally invasive approach with very small incisions. It is designed to achieve fusion at the tibiotalar joint while allowing unrestricted motion at all other joints. This benefits the patient with faster healing, faster recovery and faster loading. End-stage ankle arthritis is becoming increasingly common and can lead to significant physical disability. There are an estimated 50,000 new cases of ankle arthritis in the US each year1
“We designed our ankle fusion nail system so that patients have smaller incisions, allowing them to heal faster and function much earlier, giving them a better quality of life. The improvement in time to weight bearing for the patient is meaningful,” said Christopher Weathers, Chief Commercial Officer. “We are pleased to see our Shotel nailing system gaining traction among surgeons across the country. So far, 24 operations have been performed with it.”
Kevin Palmer, DPM, dual board certified podiatrist from Boca Raton and Delray Beach, FL, who performed the procedure, said, “The patient presented with progressively worsening ankle disease and pre-existing structurally compromised hardware. The new design of the Shotel Nail System gave me confidence that it would be stable and would hold up over the long term. During the procedure, after I removed the patient’s current hardware, the Shotel Nail System was inserted smoothly, with a smaller incision and a much less invasive approach than traditional ankle fusion systems. I also like that it provides multiple layers of compression, which is needed at the fusion site to speed healing.
“I cannot emphasize enough how important it is that the patient started walking with a CAM walking shoe within two weeks,” added Dr. Palmer added. “This will be a game changer – and life changing – for many patients in the future.”
For more information about the Shotel™ Ankle Arthrodesis Nailing System or for surgical training, visit www.shotelmedical.com.
About Shotel Medical
Shotel Medical is the developer of the Shotel™ Ankle Arthrodesis Nail System, an entirely new device that has the potential to transform patient care and have a significant impact on the healthcare landscape. Developed in collaboration with a team of biomechanical engineers, orthopedic surgeons and industry thought leaders, the device addresses the needs of patients with end-stage ankle arthritis. The unique design allows patients to heal faster, function sooner and improve quality of life compared to current treatment options. Founded in 2017, the company has offices in New Orleans and Atlanta.
About BioPro
For more than three decades, BioPro has been at the forefront of orthopedic innovation, focused on improving the lives of patients suffering from orthopedic conditions. The company is committed to developing advanced implants and surgical devices that reduce pain and restore function, providing patients and surgeons with a diverse portfolio of established solutions and emerging technology. For more information, visit https://bioproimplants.com/.
Media contact:
Barbara Bikkel
305-215-2121
369140@email4pr.com
___________________________________
1 Smyth, Niall A. MD; Dawkins, Brody J.BA; Goldstein, Joshua P.B.S.; Kaplan, Jonathan R. MD; Schon, Lew C. MD; Aiyer, Amiethab A. MD. Consumer prices for surgical treatment of ankle arthritis: limited availability and high variability. JAAOS: Global Research and Reviews 3(7):p e011, July 2019. | DOI: 10.5435/JAAOSGlobal-D-19-00011
SOURCE Shotel Medical