With more than 100,000 implants worldwide, the M6-C and M6-L drives continue to demonstrate strong clinical performance, safety and patient benefits
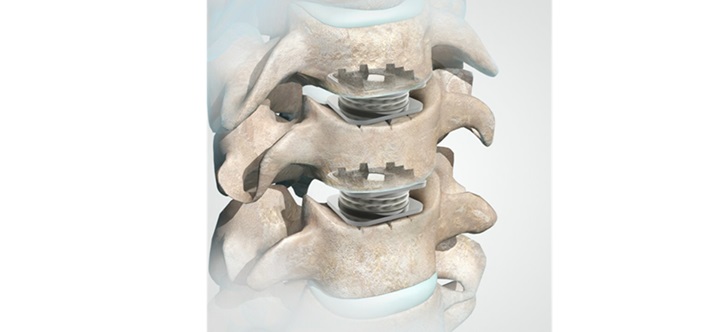
The Orthofix M6-C™ artificial intervertebral disc is a next-generation artificial intervertebral disc designed to replace an intervertebral disc damaged by degeneration of the cervical intervertebral discs. The M6-C disc is designed to restore spinal motion and is an alternative to cervical fusion.
LEWISVILLE, Texas, November 29, 2023–(BUSINESS WIRE)–Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced the release of the five-year results of its US clinical trial comparing the M6 -C™ artificial cervical disc with anterior cervical discectomy and fusion (ACDF). Published in The Spine diarypatients treated with the M6-C disc demonstrated superior clinical success at 60 months compared to ACDF patients. Secondary findings indicated significant improvements in neck and arm pain, function and quality of life. The M6-C patient group maintained flexion-extension and lateral bending motion as reported at previous time points. The release of these data coincides with the recognition that more than 100,000 implantations of the M6-C artificial cervical disc and the M6-L artificial lumbar disc (available outside the US only) have been performed worldwide since the product was first introduced in 2006.
“Publication of these data is important as it validates the strong clinical performance observed in the five-year data from the US IDE study,” said lead author Dr. Frank Phillips, professor of orthopedic surgery at Rush University Medical Center in Chicago and the director investigator for the FDA clinical trial. “Artificial intervertebral disc replacement is becoming the gold standard of care for indicated patients who might otherwise experience intervertebral disc fusion. Data from this study demonstrate that the M6-C artificial disc demonstrated superior clinical success over five years compared to ACDF controls. Furthermore, significantly more subjects in the M6-C group reported improved pain and physical functioning scores than observed in ACDF subjects, with no difference in reoperation rates or safety outcomes.”
A prospective, nonrandomized, concurrently controlled clinical trial, the M6-C IDE study, was conducted at 23 sites in the United States with a mean patient age of 44 years. The study evaluated the safety and effectiveness of the M6-C artificial cervical disc compared to ACDF for the treatment of symptomatic single-level cervical radiculopathy with or without umbilical cord compression. The overall success rate for the protocol-specified primary endpoint for the M6-C disk patients was 82.3 percent, compared to 67.0 percent in the control group. The rates of subsequent surgical intervention (SSI) on the M6-C drive were 3.1 percent, device or procedure related serious adverse events (SAE) were 3.1 percent and were comparable to ACDF rates of SSI of 5.3 percent and SAE failure of 4.8 percent. The M6-C drive received U.S. Food and Drug Administration (FDA) approval in February 2019 based on the two-year results of this study.
“To date, there have been more than 100,000 implants of M6 artificial disc technology in 20 countries around the world,” said Kevin Kenny, president of Orthofix Global Spine. “We are proud of this life-changing technology that has helped so many people enjoy their lives again.”
About the M6-C artificial cervical disc
The M6-C artificial cervical disc is designed to maintain the natural behavior of a functional spinal unit by replicating the biomechanical characteristics of the original disc and is indicated as an alternative to cervical fusion. The unique design of the M6-C disc features a compressible viscoelastic core core and annular structure construction that allows shock absorption at the implanted level, providing a controlled range of motion as the spine transitions into its combined complex movements.
Orthofix invites attendees of the Cervical Spine Research Society (CSRS) annual meeting in Las Vegas, November 29 through December 2, 2023, to visit booth #109 to learn more about the company’s full portfolio of cervical solutions .
About Orthofix
Orthofix is a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spine hardware, bone growth therapies, specialty orthopedic solutions and a leading surgical navigation system. The products are distributed in approximately 68 countries around the world.
The company is headquartered in Lewisville, Texas, where it conducts general business, product development, medical education and manufacturing, and corporate offices in Carlsbad, CA, with a focus on spine and biologics product innovation and surgeon education, and Verona, Italy. with an emphasis on product innovation, production and medical education for orthopedics. The combined company’s global R&D, commercial and manufacturing footprint also includes facilities and offices in Irvine, CA, Toronto, Canada, Sunnyvale, CA, Wayne, PA, Olive Branch, MS, Maidenhead, UK, Munich, Germany, Paris, France and São Paulo, Brazil. For more information, visit Orthofix.com.
Forward-Looking Statements
This press release may contain forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects” , ‘intends’, ‘predicts’, ‘potential’, ‘continue’ or other similar terminology. Orthofix cautions you that statements in this press release that are not descriptions of historical facts are forward-looking statements based on the company’s current expectations and assumptions. Any forward-looking statement contained in this press release is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Applicable risks and uncertainties include, but are not limited to: the ability of newly launched products to perform as designed and intended and to meet the needs of surgeons and patients, including due to the lack of robust clinical validation; and the risks identified under the heading “Risk Factors” in Orthofix Medical Inc.’s annual report. on Form 10-K for the fiscal year ended December 31, 2022, which was filed with the Securities and Exchange Commission (SEC) on March 6. , 2023. The Company’s public filings with the Securities and Exchange Commission are available at www.sec.gov. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they are made. Orthofix does not intend to revise or update any forward-looking statement contained in this press release to reflect events or circumstances occurring after the date of this press release, except as may be required by law.
Contacts
Media relations
Denise Landry
DeniseLandry@orthofix.com
214.937.2529
Investor Relations
Louisa Smith, Gilmartin Group
IR@orthofix.com