The use of stem cells in bone grafting applications continues to grow in popularity. This is partly due to aging patient populations that require more advanced materials for successful surgical outcomes. At the same time, surgeons are seeking more advanced technologies to treat these patients.
Stem cells are unique in that they can be stimulated by their environment to change, or “differentiate,” into bone cells. Some advanced synthetic bone graft materials even have the ability to stimulate stem cells to become bone cells. But what exactly is meant by the broad term “stem cells” and what benefits do they promise? This blog covers an introduction to the biology of stem cells and how stem cells can be used specifically for bone regeneration.
Introduction to stem cells
Although their clinical use remains controversial, embryonic stem cells have an astonishing power to transform into virtually any organ or tissue under specific chemical and physical conditions. These cells are called “pluripotentfrom the Latin root multi- meaning a lot of. Other types of stem cells, also called “multipotent” or “adult stem cells,” are more differentiated, meaning they can form into a more limited variety of tissues. For bone regeneration applications, adult stem cells are used can or are autologous stem cells (coming by the patient who is there treated, also known as autotransplant) or like a allograftwhich means, allogeneic stem cells (coming by another person, usually a corpse). Of course, any bone graft product containing donated allogeneic stem cells (cellular allografts) must comply with a number of FDA regulations and protocols to ensure its safety and assess its effectiveness.
One type of adult stem cell Ordinaryly associated with bone grafting applications is a mesenchymal stem cellor MSC. This cells were first identified in the middle–70s. MSCs are partially differentiated, which makes them capable of producing a variety of structural or stromal tissues, such as bones, cartilage, tendons, muscles, blood, and thick (Figure 1). They are limited to these tissue types becauseusand they Are embryological derived from by the mesoderm, hence the term “monthsenchymal.“ In contrastthe ectoderm is responsible for forms skin and the central nervous system, while the endoderm is responsible for the functional cells of the main internal organs, such as the liver, pancreas, and kidneys.
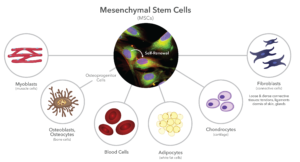
MSCs are found throughout the body, particularly pericytes that are embedded in the structural components of microvascular walls. Therefore, bone marrow is particularly rich in MSCs. MSCs are spindle-shaped, but not particularly unique in their morphology. Because they replicate easily, they grow on tissue culture plates as “colony-forming units” or CFUs. They are best identified chemically by specific antigens, called “Clusters of Differentiation”, in their cell membranes, such as CD105 and CD44. MSCs are rare, accounting for less than 0.1% of nucleated cells in the body, and they continue to decline with age. Although MSCs can replicate more than 50 times, their doubling rate is slow, more than 1-3 days, and decreases with age.
Allogeneic MSCs can be used in patients because they are thought to immune privileged. This means that they do not contain important HLA (Human Leucocyte Antigens) antigenic components in their cell membranes that the patient recipient sees as foreign. Therefore, they are not detected by the patient’s immune system when they are implanted. In contrast, HLA compatibility Is required for every organ transplant to prevent rejection. MSCs can therefore theoretically be used to treat any patient without concerns about histocompatibility.
Mesenchymal Use of stem cells in bone transplantation
Bone is one of the most easily regenerated structural tissues, so it is no surprise that MSCs are readily available in bone marrow. For this reason, bone marrow aspirate (which is harvested from cancellous bone) is widely used in combination with other bone graft materials. Due to the popularity of bone marrow-derived stem cells, tissue banks eventually found a way to process allograft bone while keeping the donor MSCs alive. Today, both mesenchymal stem cells (MSCs) and more differentiated stem cells, osteoprogenitor cells (OPCs) (Figure 1) are harvested from cadaver bone. These MSCs and OPCs are then added to DBM (demineralized bone matrix) and/or cancellous bone chips from the same donor to create a variety of allograft stem cell products.
There is much debate about the efficacy of transplanted MSCs and OPCs. It is not clear whether these cells actually survive, let alone divide into viable cells, after transplantation. However, there is data to support that they act as paracrine cells (signaling cells) by releasing cytokines and other growth factors into their environment. In this way, they indirectly stimulate bone regeneration, rather than directly differentiating into osteoblasts.
The future of stem cells in bone regeneration
Modern process techniques have very extended access to stem cells for use in surgeryFor procedures requiring bone tissue graftsurgeons are expected to use mesenchymal Rod cells, osteoancestor cells, And related technologies to grow new bone andd patients help to heal more complete. While stem cell Their use remains a topic of debate, but these cells show promise in the field of bone regeneration and will likely continue to play a role in future bone regeneration technologies.
Leave a Reply