Analysis of cohort 1 from Cochin Hospital, Paris
Study population
Between May 2016 and February 2018, a total of 101 patients (85 women, 84%) with established RA were included. These patients had a mean age of 58 ± 13 years, a mean disease duration of 14 ± 11 years, and a mean follow-up age. -from 41 ± 15 months. Positive rheumatoid factors and anti-CCP antibodies were detected in 80 (79%) and 83 (82%) patients, respectively. Erosions were present in 63 (62%) patients; 70 patients (69%) received corticosteroids (including 9 at a dose > 10 mg/day), 78 received conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), including 61 (60%) with MTX, and 59 (58%) received targeted biological DMARDs (bDMARDs). During the inclusion visit, 13 patients initiated a first-line bDMARD or switched to another bDMARD due to inadequate disease control. Detailed characteristics of our study sample are shown in Table 1.
Results
The number of annual consecutive visits ranged from 2 to 5 (88 patients with 3 visits, 72 with 4 visits, and 65 with 5 visits). Disease flares occurred in 38 patients during the mean follow-up period of 41 ± 15 months. Of these 38 patients, targeted therapy was added or modified in 26 patients due to inadequate disease control: 10 started on a bDMARD or a targeted synthetic (ts)-DMARD and 16 switched from a bDMARD to a new b- or tsDMARD (Table S1) . The mean time to treatment adaptation was 35 ± 13 months.
Primary endpoint: evaluation of the predictive value of SEMA4A for the occurrence of treatment failure
Baseline SEMA4A levels > 94 ng/ml were predictive of treatment failure, defined by the occurrence of flares AND treatment escalation (n = 26 patients), with an HR of 2.73 (95% CI 1.24 –5.96) (Fig. 1A). Results were unchanged after excluding the 13 patients with active disease at baseline who requested addition or change to a bDMARD (HR: 2.83, 95% CI 1.14–7.52).
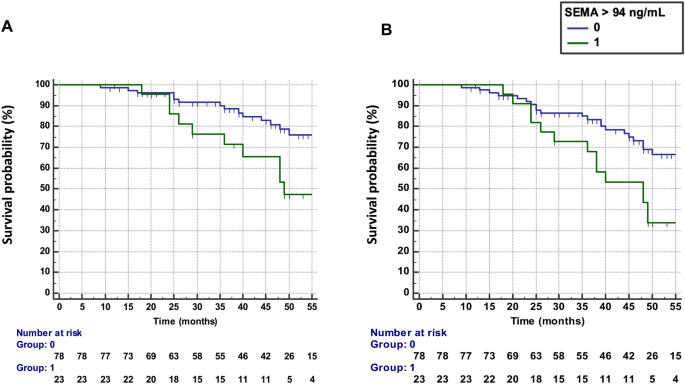
Predictive value of SEMA4A for the progression of rheumatoid arthritis in Paris cohort 1. (a) Time to treatment failure (defined as flares AND treatment escalation) according to circulating SEMA4A concentrations (≤ or > 94 ng/ml). (b) Survival without disease flare according to circulating SEMA4A concentrations (≤ or > 94 ng/ml).
Secondary endpoints
Elevated SEMA4A levels (>94 ng/ml) at baseline were predictive of flare occurrence (n = 34 patients) during the follow-up period (Fig. 1B) with a hazard ratio (HR) of 2.43 (95% confidence interval ). , CI 1.27–4.68). Results were unchanged after excluding the 13 patients with active disease at baseline (HR 2.36, 95% CI 1.15–4.89).
Baseline SEMA4A concentrations were significantly increased in patients who experienced flares during the follow-up period (78 ± 30 ng/ml vs. 60 ± 24 ng/ml, p < 0.001) (Fig. 2A). SEMA4A levels were also significantly higher in the 13 patients with active disease at baseline who requested the addition or modification of a bDMARD, compared with the 88 patients on stable treatment (84 ± 33 ng/ml vs. 63 ± 26, p = 0.011). Although baseline SEMA4A concentrations were higher in patients experiencing flares AND treatment escalation compared to those on stable treatment (75 ± 31 ng/ml vs. 63 ± 26 ng/ml, p = 0.060), this did not reach significance (Fig. 2B). Patients with elevated SEMA4A levels at baseline maintained higher DAS28 levels throughout the follow-up period, with significant differences at visits 1, 2, and 5 (Fig. 2C).
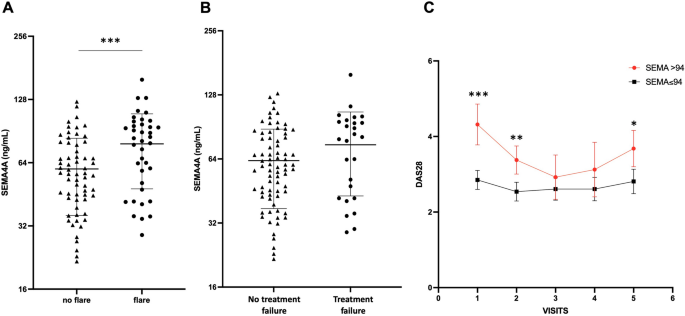
Baseline circulating SEMA4A levels according to the occurrence of (a) disease flare or (b) treatment failure (defined as flares AND treatment escalation) during the prospective follow-up period in Paris cohort 1. (c) Course of the DAS28 during the follow-up period according to baseline SEMA4A concentrations (≤ or > 94 ng/ml). All data are presented as the mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, determined by Student's t-test.
Integration of SEMA4A with other predictors of treatment failure
A baseline DAS28 > 3.2 (HR 2.17, 95% CI 1.01–4.72) and the presence of active synovitis, defined by at least grade 2 Doppler activity8, detected at at least one joint on power Doppler ultrasound (PDUS) (HR 3.60, 95% CI 1.07–12.15) were predictive of further treatment failure. These results were not changed after excluding the 13 patients with active disease at baseline.
Baseline age, disease duration, ACPA or RF positivity, smoking status, presence of erosions, series of targeted DMARDs, corticosteroid treatment, and CRP levels were not predictive of treatment failure (Table 2). Multivariate Cox analyzes adjusted for these covariates confirmed that SEMA4A was the only independent predictor of treatment failure (HR 2.71, 95% CI 1.14–6.43).
SEM4A was also confirmed as an independent predictor of flares, along with DAS28 and synovial hyperhemia (Table 2).
We then assessed the possible combination of DAS28, PDUS and SEMA4A concentrations to predict the occurrence of treatment failure and flares (Table 3). The combination that provided the best predictive value was a DAS28 > 3.2 and/or presence of active synovitis on PDUS and/or SEMA4A concentrations > 94 ng/ml (HR 10.42, 95% CI 1.41–76 .94 for treatment failure and 4.88, 95% CI 1.50–15.89 for flares) (Fig. 3A,B). Matrix models also highlighted the ability of the combination of these 3 parameters to predict the occurrence of treatment failure and flares (Fig. S1): Treatment failure and flares of RA occurred in 53% and 73% of patients with DAS28 > 3.2 at baseline and the presence of active synovitis at PDUS and SEMA4A concentrations > 94 ng/ml, respectively. Furthermore, only one patient with a DAS28 ≤ 3.2, no active synovitis and SEMA4A ≤ 94 ng/ml experienced treatment failure and RA attacks.
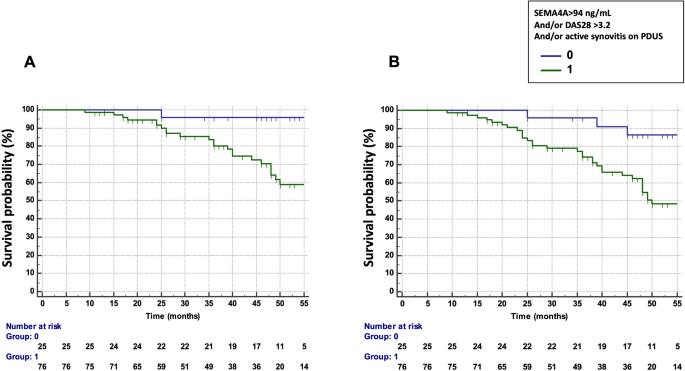
Predictive value of SEMA4A, alone or in combination with a Disease Activity Score (DAS) 28 > 3.2 and/or the presence of active synovitis on power Doppler ultrasound (PDUS) in cohort 1 from Paris. (a) Time to treatment failure (defined as flares AND escalation of treatment) according to circulating SEMA4A concentrations (> 94 ng/ml) and/or a DAS28 > 3.2 and/or the presence of active synovitis on PDUS. (b) Survival without disease flare according to circulating SEMA4A concentrations (> 94 ng/ml) and/or a DAS28 > 3.2 and/or the presence of active synovitis on PDUS.
Predictive value of SEMA4A in the subgroup of patients with low disease activity or remission
Among the 58 patients with a DAS28 < 3.2 at baseline, treatment failed in 11 (19%) patients during the observation period. In this population, increased SEMA4A concentration was the only variable predicting the occurrence of treatment failure (HR 3.50, 95% CI 1.02–12.01). The presence of active synovitis detected on at least one joint on PDUS and other clinical or biological variables did not predict treatment failure (Table S2).
In the 37 patients with a DAS28 < 2.6, treatment failure occurred in 4 patients (11%) and elevated SEMA 4A showed a trend for predicting treatment failure (HR 3.30, 95% CI 0.82–152.11, p = 0.069).
Elevated SEMA4A concentration was also identified as the only predictor of flares (n = 16, 28%) in this subgroup of 58 patients with DAS28 < 3.2 (HR 3.68, 95% CI 1.33–10.17 ).
Analysis of cohort 2 from Pelegrin Hospital, Bordeaux
Study population
A total of 40 patients (29 women, 73%) were included. These patients had a mean age of 57 ± 14 years, a mean disease duration of 5 ± 6 years, and active disease with a mean DAS28 of 5.12 ± 1.40. Positive rheumatoid factors and anti-CCP antibodies were detected in 27 (79%) and 28 (82%) patients, respectively. Erosions were present in 16 (40%) patients; 26 patients (65%) received corticosteroids. During the inclusion visit, 15 patients started MTX as first-line treatment and 25 started tocilizumab. Tocilizumab initiators were older, had longer disease duration and disease activity, and received corticosteroids more often than MTX initiators. Detailed characteristics of our study sample are given in Tables 1 and S3.
Analysis of the course of SEMA4A serum levels according to response to treatment
Of the 40 patients included, 4 experienced no response to treatment, 10 had a moderate response and 26 had a good response. As previously observed, baseline SEMA4A levels correlated with the DAS28 (r = 0.29, p = 0.038) and a trend was observed with CRP (r = 0.26, p = 0.10). At month 3, SEMA4A concentrations correlated with DAS28 and CRP (r = 0.31, p = 0.029 and r = 0.38, p = 0.017, respectively). Furthermore, baseline SEMA4A concentrations were significantly increased in active patients at inclusion, defined by a DAS28 > 3.2 (Fig. S2A). Interestingly, baseline SEMA4A levels were significantly higher in patients who otherwise experienced no or moderate response (198 ± 30 ng/ml) compared to patients with a good response (176 ± 24 ng/ml, p = 0.035) (Fig. S2B ). It was found that serum SEMA4A levels decreased significantly between m0 and m3, especially in the group of patients with good clinical response (Fig. S2C). This result was observed in the subgroups of patients starting MTX or tocilizumab (Fig. S2D,E).
Leave a Reply