Trial design
This controlled pilot clinical study involved 60 patients admitted to Ghaem Hospital of Mashhad, Iran. These patients were specifically from the cardiac surgery intensive care unit and were admitted between May 2020 and January 2021 (Figure 1).
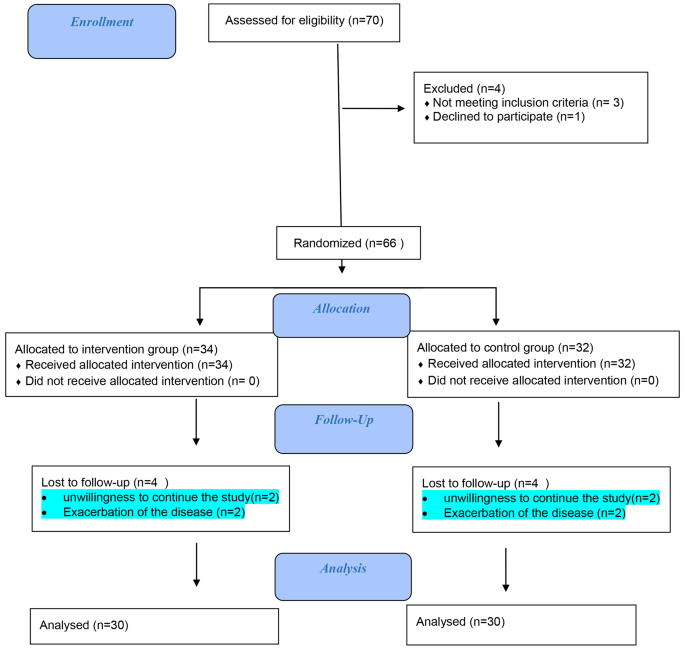
CONSORT Flowchart of participants
Attendees
The study included patients who met specific inclusion criteria. These criteria required that patients be between 18 and 60 years old and willing to undergo non-emergency coronary transplant surgery. On the other hand, the exclusion criteria included patients who experienced loss of consciousness until the day after surgery, those who did not have a smartphone, individuals with severe postoperative arrhythmias and hemodynamic disorders, and patients who were prohibited by their physicians from participating in rehabilitation.
Intervention
Software production
Prior to the design of the software, extensive research was done to prepare its contents. This involved reviewing various texts, including articles, reference works, and gathering insights from experienced nurses in specialist care units. The content was then submitted to a panel of ten specialists for validation, and their suggested revisions were incorporated.
The software’s educational content covered a range of topics, including breathing and diaphragm exercises, instructions on physical exercises and their proper performance, discussions and interactions with patients, and encouragement for patients to participate in routine activities. These concepts were presented primarily through instructional videos and engaging animations.
Once the content was ready, it was handed over to the software development and information technology team for creating the software. After the initial software was developed, a specialized validation process was carried out by ten IT experts to ensure its functionality and effectiveness.
To validate the software, both white-box and black-box testing methods were used. In black-box testing, users without knowledge of the software’s internal structure enter the desired items and verify the recorded information. The purpose is to ensure accurate data recording. White-box testing, on the other hand, requires users to have knowledge of the software’s internal structure and is typically performed by designers or experts. For example, to assess the speed of the software, several items were selected at different speeds and the accuracy of the selections was examined.
The next phase included compatibility testing and security testing. Compatibility testing involved installing the application on multiple Android smartphones and tablets to assess performance on each device. In the security testing, a double confirmation method was implemented to ensure accurate recording of each patient’s problems. This required the patient to confirm the selected item by clicking again, which reduced the chance of inadvertent data entry errors.
The augmented reality software is registered and approved within the electronic services system of the Information Technology Organization of Iran.
To evaluate patient satisfaction with the augmented reality software, the Mobile Application Rating Scale (MARS) was used.
This scale evaluates the quality and performance of the application on four dimensions: attractiveness (5 questions), functionality (4 questions), aesthetics (3 questions), information (7 questions) and subjective quality (4 questions). Each item in the scale was rated on a five-point scale. The maximum achievable score was 115, while the minimum acceptable score was set at 23. For a detailed presentation of the results, please refer to (Table 1).
Phase I cardiac rehabilitation training based on augmented reality
After establishing the necessary agreements with officials at Ghaem Hospital in Mashhad, Iran, the first author of the study initiated the sampling process. In the intervention group, rehabilitation program training began upon patient entry into the cardiac surgery intensive care unit and continued until the patient’s discharge.
During several sessions, augmented reality software was used to train patients in physical activities, such as walking around the hospital ward and climbing stairs. These exercises were performed under the direct supervision of the researcher and were taught individually to each patient using the augmented reality software. The duration of physical activity varied depending on the patient’s condition and the length of hospital stay, ranging from 5 to 10 minutes. During the rehabilitation sessions, the ECG and perceived exercise intensity were closely monitored and controlled.
In the control group, the rehabilitation training program was implemented using a routine method based on the Ministry of Health protocol. The researcher provided face-to-face training within the unit. Both the intervention and control groups completed the cardiac self-efficacy questionnaire upon admission and discharge to the special care cardiac surgery department.
Results
The data collection process used two demographic information questionnaires and a cardiac self-efficacy questionnaire.
The cardiac self-efficacy questionnaire used in this study was the Cardiovascular Management Self-Efficacy Questionnaire, which was developed by Estka of Italy in 2015. This questionnaire consists of 9 questions, each rated on a 5-point Likert scale, ranging from ‘completely confident’ to ‘not at all confident’. The questionnaire consists of three subscales.
The first four questions assess a person’s belief in their ability to quit smoking, maintain good nutrition, exercise, and avoid stressful situations. This subscale is called cardiac risk factor self-efficacy. Questions 5 and 6 relate to a person’s confidence in remembering to take medications correctly, which reflects self-efficacy for medication adherence. Finally, questions 7 through 9 evaluate a person’s belief in their ability to identify symptoms and signs of disease worsening, indicating self-efficacy in recognizing symptoms.
Each answer is assigned a score, with ‘not at all confident’ given a score of one, ‘somewhat confident’ given a score of two, ‘somewhat confident’ given a score of three, ‘fairly confident’ given a score of four, and “completely confident” with a score of five. Total scores range from 9 to 45, with higher scores indicating greater self-efficacy in cardiovascular management [21]. Borzou et al. (2017) evaluated the validity and reliability of this tool in Iran [33]. Patients completed the Cardiovascular Management Self-Efficacy Questionnaire both before and after the intervention.
Sample size and randomization
The study involved the continuous and purposeful selection of patients who were then randomly assigned to one of two groups. After confirming that they met the inclusion criteria, eligible individuals were divided into intervention and control groups using a random sequence generated by SPSS software. This series was kept in a sealed envelope to ensure confidentiality. Although it was challenging to blind the participants in this study, the outcome assessors and statisticians were unaware of the type of intervention, ensuring a level of objectivity.
Because no comparable study was found examining the effectiveness of phase I cardiac rehabilitation training based on augmented reality on the self-efficacy of patients undergoing coronary artery bypass surgery, a sample size of 10 participants was determined for each group. The sample size was calculated using the mean comparison formula, with a 95% confidence interval and 80% test power for each group, resulting in a total of 20 participants. To account for the potential dropout rate, an additional 30 participants were added to each group, representing a 10% increase over the values calculated in the formula.
$$N = \text \left( Z1 – \alpha /2\text + \text Z1 – \beta \right)2\text \left ( S12\text + \text S22 \right)/\left( X1 – X2 \right)2$$
$$Z_1 – \alpha /2 = \text 196$$
$$Z_1 – \beta = \text 0.85$$
$$X_2 = \text 8.3$$
statistical methods
After data collection and sampling, the collected data was analyzed using SPSS 21. Various statistical tests were used, including the independent t-test, the Mann-Whitney test, the paired t-test, and the chi-square test. These tests were performed at a 95% confidence level to ensure statistical significance. Descriptive indicators such as mean, standard deviation and frequency were also used to provide a comprehensive overview of the data. Cohen’s d was also used to evaluate the magnitude of the effect size, calculated by standardized mean difference, with g > 0.2 to 0.5 = small effect size, g > 0.5 to 0.8 = medium effect size, and g > 0 .8 = large effect size [38].

Leave a Reply