Porter Hospital becomes first facility in Denver to give patients with chronic low back pain access to revolutionary Nociscan technology that measures biomarkers to pinpoint the source of pain
George Frey, MD becomes the first Aclarion KOL surgeon to use in-hospital MRI to add Nociscan data to his treatment decision process for patients with chronic low back pain
Published clinical data shows that patient outcomes improve and costs decrease when surgical treatment is consistent with disc levels that Nociscan identifies as painful
BROOMFIELD, CO, October 25, 2023 (GLOBE NEWSWIRE) — via NewMediaWire – Aclarion, Inc., (“Aclarion” or the “Company”) (Nasdaq: ACON, ACONW), a healthcare technology company that uses biomarkers and proprietary enhanced intelligence algorithms to help physicians identify the location of chronic low back pain, today announced their expanded presence in Colorado with AdventHealth Porter. From their Denver location, AdventHealth Porter provides exceptional medical care to patients throughout Colorado’s frontline region.
George Frey MD, orthopedic surgeon and founder of the Colorado Comprehensive Spine Institute said, “Treating patients effectively starts with an informed understanding of where their pain comes from. In my experience with Nociscan, objectively measuring the biomarker content associated with pain in an intervertebral disc that may look healthy on an MRI adds to my knowledge of how to treat that patient. At AdventHealth Porter, we strive to provide cost-effective care of the highest quality. The published evidence makes it clear to me that adding Nociscan to our diagnostic evaluation can help us do just that.”
Brent Ness, CEO of Aclarion, said: “In line with our strategy to bring Nociscan to a standard of care through KOL advocacy, we applaud AdventHealth Porter for bringing Nociscan technology to Denver in support of Dr. Frey to use our technology to improve results. while reducing costs. The support of pioneering leaders like Dr. Frey and AdventHealth is exactly the leadership we need to unequivocally demonstrate the superior clinical outcomes for patients that will drive payers’ coverage decisions and provide all patients with access to Nociscan technology. We thank Dr. Frey and AdventHealth look forward to the positive impact that increasing Nociscan volumes will have on the surgical outcomes of chronic low back patients in and around the Denver community.”
“Providing exceptional care with cutting-edge treatments is a core value of our multidisciplinary care team here at AdventHealth Porter,” said Carol Bermingham, Imaging Manager at AdventHealth Porter. “We are excited about adding Nociscan to our MRI capabilities and believe that providing personalized biomarker data to treating physicians is one more investment in our commitment to providing an environment where our physicians can do their very best for their patients . Our radiology service line always strives to stay at the forefront of the technical advancement curve.”
Chronic low back pain (cLBP) is a global healthcare problem, with approximately 266 million people worldwide suffering from degenerative spine disorders and low back pain. It is estimated that low back pain affects approximately 30 million adults in the U.S. population annually, leading to millions of doctor consultations each year.
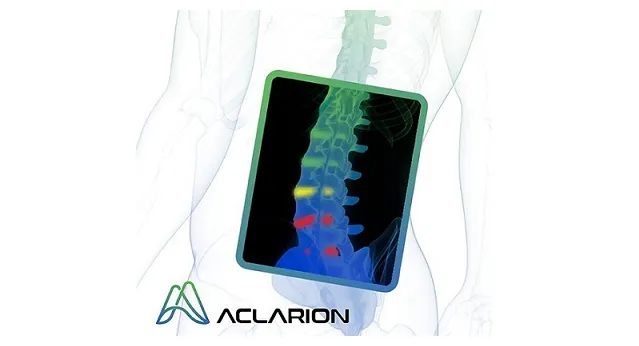
Aclarion’s proprietary decision support tool, Nociscan, is the first evidence-based SaaS platform that helps physicians non-invasively distinguish between painful and non-painful discs in the lumbar spine. Nociscan objectively quantifies chemical biomarkers shown to be associated with disc pain. Biomarker data is fed into proprietary algorithms to indicate whether a disc may be a source of pain. When combined with other diagnostic tools, Nociscan provides critical insights into the location of a patient’s low back pain, giving clinicians clarity to optimize treatment strategies.
About Aclarion, Inc.
Aclarion is a healthcare technology company that uses magnetic resonance spectroscopy (“MRS”), proprietary signal processing techniques, biomarkers and enhanced intelligence algorithms to optimize clinical treatments. The company is entering the chronic low back pain market for the first time with Nociscan, the first evidence-based SaaS platform that helps physicians non-invasively distinguish between painful and non-painful discs in the lumbar spine. Through a cloud connection, Nociscan receives magnetic resonance spectroscopy (MRS) data from an MRI machine for each lumbar disc being evaluated. In the cloud, proprietary signal processing techniques extract and quantify chemical biomarkers shown to be associated with disc pain. Biomarker data is fed into proprietary algorithms to indicate whether a disc may be a source of pain. When combined with other diagnostic tools, Nociscan provides critical insights into the location of a patient’s low back pain, giving clinicians clarity to optimize treatment strategies. For more information, please visit www.aclarion.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 about the Company’s current expectations about future results, performance, prospects and opportunities. Statements that are not historical facts, such as “anticipates,” “believes” and “expects” or similar expressions, are forward-looking statements. These forward-looking statements are based on management’s current plans and expectations and are subject to a number of uncertainties and risks that could materially affect the company’s current plans and expectations, as well as its future results of operations and financial condition. These and other risks and uncertainties are discussed in more detail in our filings with the Securities and Exchange Commission. Readers are encouraged to read the section entitled “Risk Factors” in the Company’s April 21, 2022 Prospectus as filed with the Securities and Exchange Commission on April 25, 2022 under Rule 424(b)(4), as well as other disclosures. included in the Prospectus and subsequent filings with the Securities and Exchange Commission. Forward-looking statements in this announcement are made as of this date and the Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Investor contacts:
Kirin M. Smith
PCG Advice, Inc.
646.823.8656
ksmith@pcgadvisory.com
Media contacts:
Jodi Lamberti
SPRIG advice
612.812.7477
jodi@sprigconsulting.com