Health benefit of patients with established rheumatoid arthritis and its influencing factors: a multicenter study in China
Cross-sectional data of RA patients in the intermediate and advanced stages from 8 tertiary hospitals in four provincial capitals Nanjing, Hangzhou, Chengdu and Shijiazhuang (two of each) were obtained, using the Chinese version of the EQ-5D-5L to measure and rate HRQOL. The patients were enrolled by trained investigators based on quota sampling from June to July 2020.
Participants in the study
Based on available sources and rules of thumb, a total of 200 patients (50 in each city, male/female = 1:2) were planned for recruitment. The inclusion criteria were: (1) Informed and voluntary; (2) 18-70 years (Given the challenges and precision associated with completing questionnaires by older people, previous research protocols26and available sources); (3) Diagnosed with RA according to the diagnostic criteria of the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification (score ≥ 6)27The exclusion criteria were: (1) pregnant women, persons with psychiatric disorders, and patients who were unconscious and unable to express their condition; (2) patients suffering from other serious illnesses such as tumors and myocardial infarction.
Data collection
A total of 16 trained interviewers divided into 8 groups were responsible for data collection in each of the 8 hospitals. In the corresponding departments of each hospital during the study (no distinction between outpatients and inpatients), the interviewers introduced the aim and content of the study to the patients and their attending physicians and obtained written informed consent. Then, the consenting patients and physicians were asked to fill out the respective questionnaires independently in a quiet room. If patients have questions about the questionnaire, the interviewers should provide explanations, but they should not guide or actively hinder the patients in filling out the questionnaire. The completed questionnaires were reviewed by the interviewers to identify any obvious errors or missing data. After the questionnaires were retrieved, the data were digitalized and reviewed by 2 independent and transparent auditors.
Questionnaires for patients and physicians
Two respective questionnaires for the patients and physicians were provisionally designed based on the literature15.28. Then, based on the opinions of experts, we revised the questionnaires and conducted a pilot survey in 2 tertiary hospitals in Nanjing to validate the rationality, readability, and comprehensibility of the questionnaires. (Since the subjective questions in the questionnaire are all derived from validated scales, we did not repeat the validation of the psychological measurement properties of the questionnaire.) The questionnaires were revised and formed the final version based on the results of the pilot survey and the suggestions of experts based on the results of the pilot survey. The rationality, readability, and comprehensibility of the questionnaire were confirmed by the experts and supported by the pilot survey.
The patient questionnaire consists of two parts. Part one collected patients’ HRQOL measured by the Chinese version of the EQ-5D-5L and other self-reported outcomes, including general health, arthritis pain, and disease activity. General health was evaluated using a four-level scale: good, general, poor, and very poor. Arthritis pain and disease activity were assessed using two visual analog scales (VAS), respectively. The patient’s assessment of arthritis pain VAS (PtAAP-VAS)29 measured the degree of pain they experienced on the day of the examination: 0 means no pain, 100 means the most severe pain. The patient’s global assessment of disease activity VAS (PtGADA-VAS)30,31 assessed current disease activity: 0 means patients feel very well and have no symptoms, 100 means patients feel very bad and have severe symptoms. Part two used questions to assess patients’ demographic characteristics, including gender, age, ethnicity, height, weight, region, marital status, education level, occupation, types of health insurance, and personal annual income.
The physician questionnaire assessed the patients’ clinical characteristics and clinician-reported outcomes, including disease stage (based on the results of joint X-ray examination, Grade I/early stage: no bone-destructive changes; Grade II/middle stage: osteoporosis with mild cartilage damage; Grade III~IV/advanced stage: osteoporosis with cartilage or bone destruction and joint deformity)32treatment modalities (inpatient/outpatient), erythrocyte sedimentation rate (ESR) (unit: mm/h), high-sensitivity C-reactive protein (CRP) (unit: mg/L), symptoms and functional capacity, swollen joints (SJC) and tender joints (TJC). Of these, symptoms and functional capacity were assessed using the Physician’s global assessment of disease activity VAS (PhGADA-VAS)30,31 (0 and 100 mean that patients are asymptomatic and normal activities are not limited, and have severe symptoms that cannot be tolerated and are unable to perform normal activities). SJC, TJC, CRP and ESR were used to calculate the 28 joint counts (DAS28) scores, including DAS28-CRP score and DAS28-ESR score33Higher DAS28 scores indicate higher disease activity. Disease activity can be divided into four states, including remission (DAS28 scores < 2.6), low activity group (2.6 ≤ DAS28 scores < 3.2), moderate activity group (3.2 ≤ DAS28 scores < 5.1), and high activity group (DAS28 scores ≥ 5.1)33.
EQ-5D-5L
Compared with EQ-5D-3L, EQ-5D-5L has improved measurement properties in terms of health status descriptive system and HUV34; and its reliability and validity have been verified in China35The descriptive system assesses the subjects’ health status on the day of the survey on five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each using five severity levels (no problems, mild problems, moderate problems, severe problems, and unable/extreme problems) and produces a total of 3125 (55) health conditions34. Each health condition can be expressed by a 5-digit number. For example, “12.345” means no difficulty with mobility, mild difficulty with self-care, moderate difficulty with usual activities, severe pain/discomfort, and extremely anxious/depressed. All health conditions defined by the system can be converted into HUVs using a value set. In this study, Chinese version of the EQ-5D-5L and the value set36 for China was adopted. The EQ-5D-5L also includes the EQ visual analogue scale (EQ-VAS) which assesses subjects’ self-reported health status via a straight line (0: the worst health you can imagine; 100: the best health you can imagine)34.
Data analysis
Descriptive statistics
Descriptive statistics such as mean and standard deviation (SD) for continuous variables, frequency and percentage for categorical variables were used to present the characteristics and outcomes of the patients. The distribution of EQ-5D-5L data (i.e., responses to each dimension, HUV, and VAS score) of the patients with different characteristics were presented. The skewness and kurtosis of EQ-5D-5L HUV were also shown. We also compared the mean age (P> 0.1) and sex ratio (P< 0.01) of patients with that of Chinese RA patients15,37,38and the EQ-5D-5L data weighted by the sex ratio of Chinese RA patients37.
Univariate analysis
To identify significant factors of the patients’ HUV, Kruskal-Wallis test or univariate regression were performed on the categorical variables or continuous variables, respectively. The variables included patient characteristics and patient/clinician-reported outcomes, of which age, body mass index (BMI), and the person’s annual income were included as categorical variables. Kruskal-Wallis test was also performed on the patients’ responses to EQ-5D-5L.
Multivariable analysis
The beta model was used for the multivariable regression analysis due to the characteristics of HUV distribution (i.e., non-normally distributed and censored at (1). The beta model required the value of the dependent variable to be between 0–1. The EQ-5D-5L scores were adjusted via the formula: adjusted score = (original score + 0.391)/1.391 (The range of EQ-5D-5L score was -0.391 to 1 based on the Chinese value set. If the original score was -0.391 or 1, the adjusted score was added or subtracted e−12 to ensure that it fell between 0 and 1.) Since the sex ratio in the sample was different from that of Chinese RA patients, we used the sex weighting in the regression. The dependent variable was the EQ-5D-5L HUV. Referring to the recommendations of clinical experts, literature15,16,17and the results of univariate analysis, some of the characteristics and outcomes were included as explanatory variables in the regression analysis. To reduce multicollinearity among the variables, Spearman rank correlations were used to test the correlations among them (correlation coefficients: very weak = 0–0.19; weak = 0.20–0.39; moderate = 0.40–0.59; strong = 0.60–0.79; and very strong = 0.80–1.00). For a few variables with the coefficient higher than 0.4, the variable that was also more correlated with other variables would be excluded (Supplementary material Appendix 1). The variables entered into the final model are related to the EQ-5D-5L score, but the correlation among these variables is low, including demographic variables age, gender, BMI, and place of residence; and clinical variables PtGADA-VAS, DAS28-ESR, disease stage and treatment modalities (the correlation heatmap is shown in Fig. 1). Finally, we would measure the multicollinearity in the regression model via the variance inflation factor (VIF).
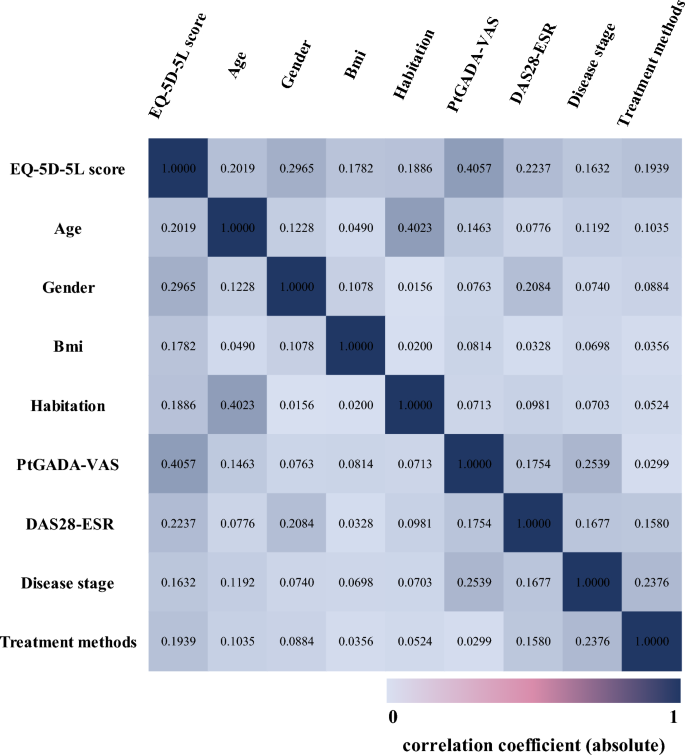
The correlation heatmap of variables.
All of the above analyses were performed on Microsoft® Excel 2021 and stata15.
Ethical approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Clinical Trial Ethics Committee. Clinical Trial Ethics Committee of Huashan Hospital Affiliated to Fudan University (Reference number 2019–252). Written informed consent to participate was signed by all participants.